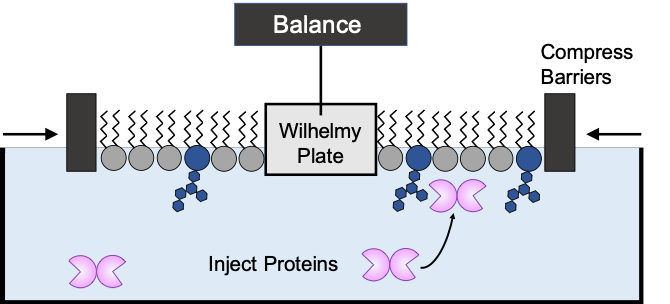
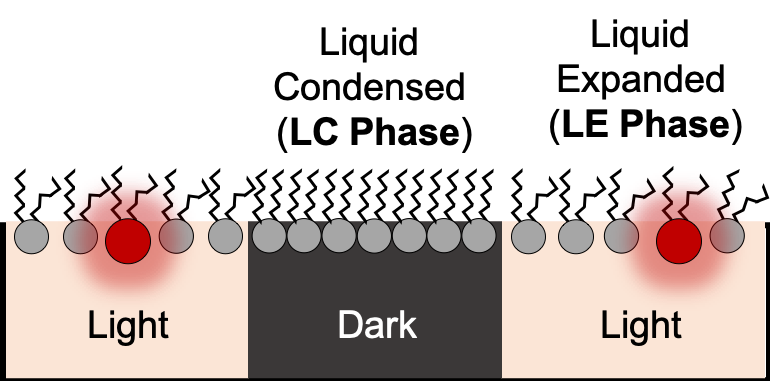
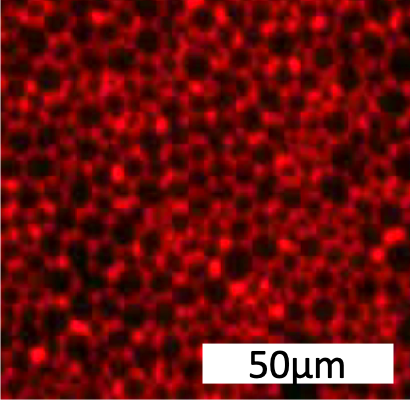
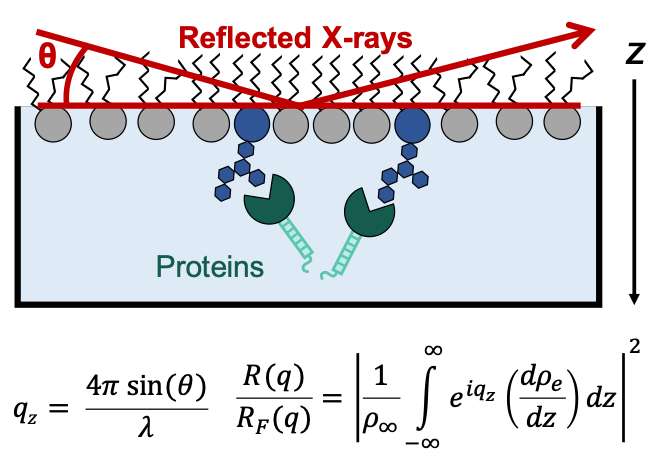
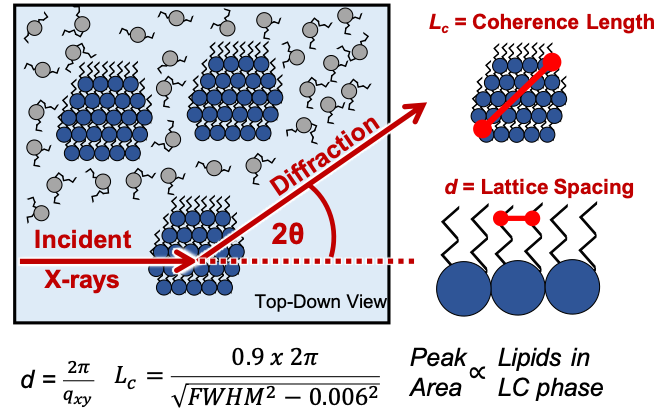
70% of pharmaceutical drugs bind membrane protein targets...
|
Yet, knowledge of membrane structure lags far behind what is known about proteins and nucleic acids.
In our lab, we use biophysical techniques to study the structure of protein/membrane interactions. |
Methods
Hover over an image to learn more!
Projects
|
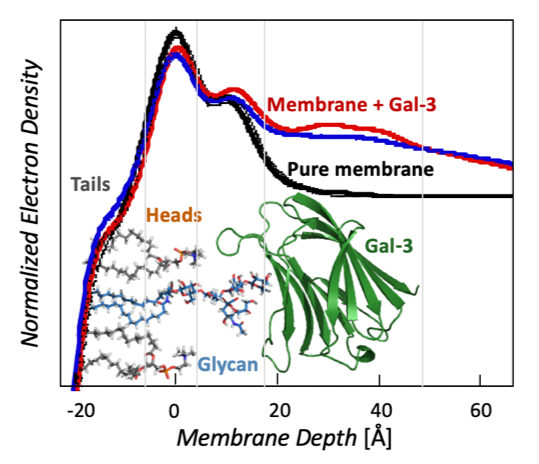
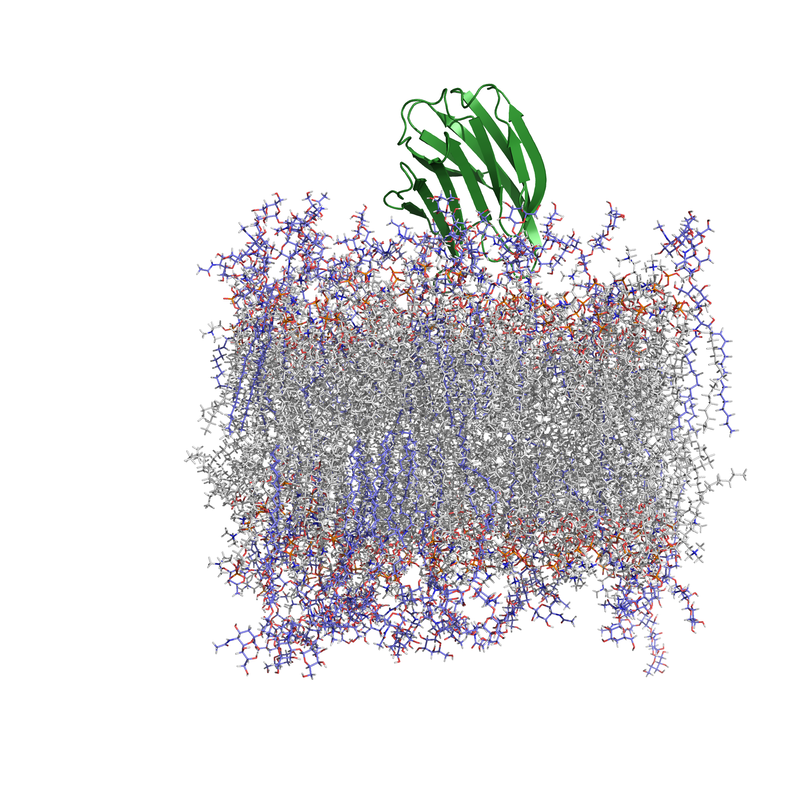
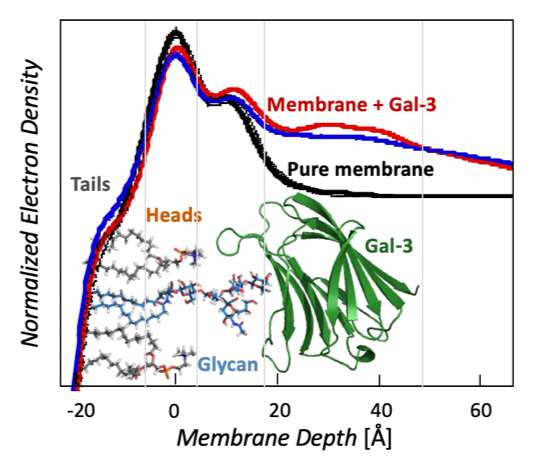
Galectin Proteins & Interactions with Glycolipids
Molecule clustering at the cell surface is critical for controlling cell signaling and behavior, and ~70% of drugs bind membrane protein targets. Currently, most drugs are designed around inhibiting a specific transmembrane receptor, but there is untapped potential to target molecule clustering to control cell behavior. Galectin proteins are thought to regulate molecule clustering because they bind and assemble a variety of β-galactoside-decorated lipids and proteins. Galectins are hypothesized to direct glycan organization on the cell surface, which leads to different cell signaling outcomes. However, the foundational principles of galectin/glycan interactions and their relationship to membrane organization are unknown, and this is an unexplored target for controlling cell behavior. The long-term goal of this project is to understand galectin-mediated mesoscale clustering of glycolipids and glycoproteins at the cell surface, which is foundational for controlling cell behavior. Funding: UCCS Undergraduate Research Award to Danielle Browne. NIH 1R15GM150123-01 “Galectin-3 and engineered variants for clustering glycolipids and glycoproteins on membrane surfaces.” NIH RePORTER |
|
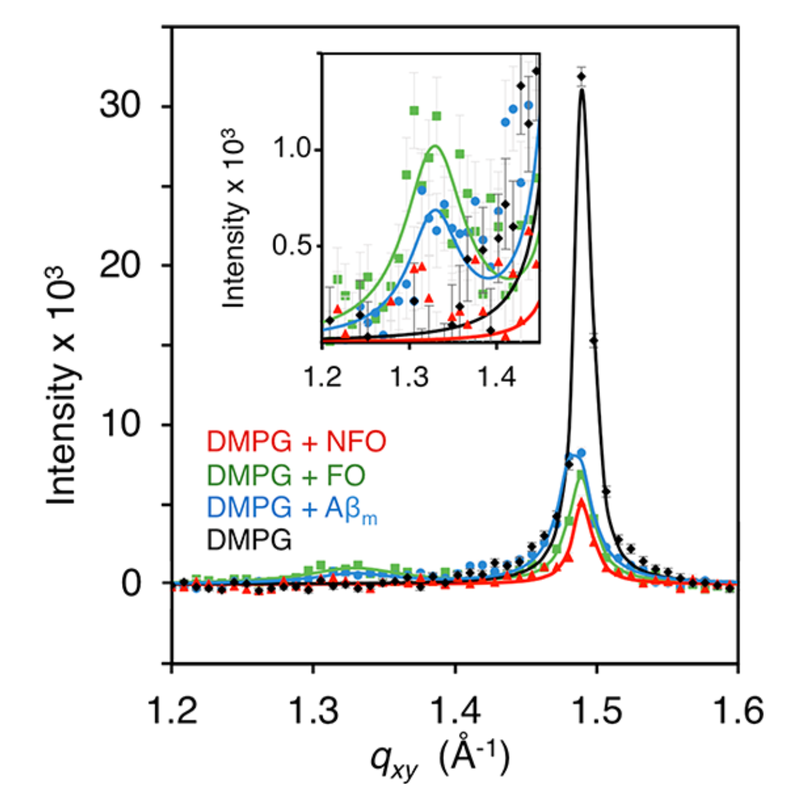
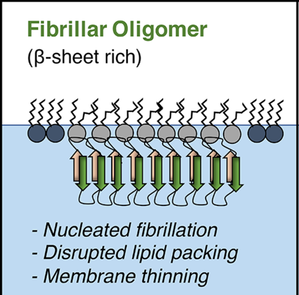
Amyloidogenic Proteins & Membrane Interactions
One major hypothesis for the cause of Alzheimer’s disease is that amyloid beta (Aβ) and tau, which are responsible for plaques and tangle in Alzheimer’s brains, interact with cell membranes. This interaction is thought to cause pore formation in the membrane, which kills neurons and leads to the classic neurodegeneration seen in Alzheimer’s disease. In the early 2000’s, researchers identified that people living in eastern Asian countries had lower incidences of Alzheimer’s disease, and this was eventually linked to compounds isolated from their diet. Our research goal is to determine how curcumin and EGCG, compounds isolated from turmeric and green tea respectively, interact with cell membranes to provide neuroprotection in Alzheimer’s disease. These compounds have been studied in several clinical trials for Alzheimer’s disease, however the foundational mechanism for their action remains unknown. Outside of Alzheimer's disease, the broader implications are to determine the interactions driving amyloidogenic proteins to interact with membranes, and determine the mechanism behind compounds inhibiting these interactions. Funding: UCCS Undergraduate Research Awards to Billy Stone and Advita Bhatia |
|
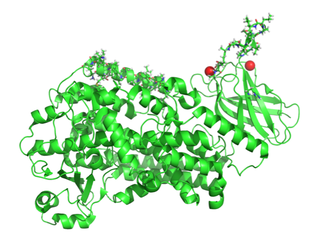
Membrane-Bound Form of Lipoxygenase
Lipoxygenases are a family of enzymes that selectively oxidize poly-unsaturated fatty acids. The lipoxygenase enzyme 15-LOX-2 has been linked to the development of atherosclerotic plaques due to oxidation of PUFAs in the membrane. This activity triggers increases oxidized lipids in LDL, which are ingested by macrophages, eventually leading to accumulation of foam cells and plaque development beneath the endothelium of blood vessels. 15-LOX-2 is expected to bind membranes through peripheral interactions driven by a surface-exposed hydrophobic loop. If the membrane-bound structure of the protein was determined, then this information would build the foundation for making 15-LOX-2 a druggable target for mitigating heart disease. Funding: NIH 1R15GM143724-01 Sub-award to UCCS “Conformational Flexibility of Lipoxygenases and its Role in Regulation and Substrate Acquisition.” (PI: Nathan Gilbert, Louisiana State University) NIH RePORTER |